
How Chlorine Dioxide Works at the Molecular Level
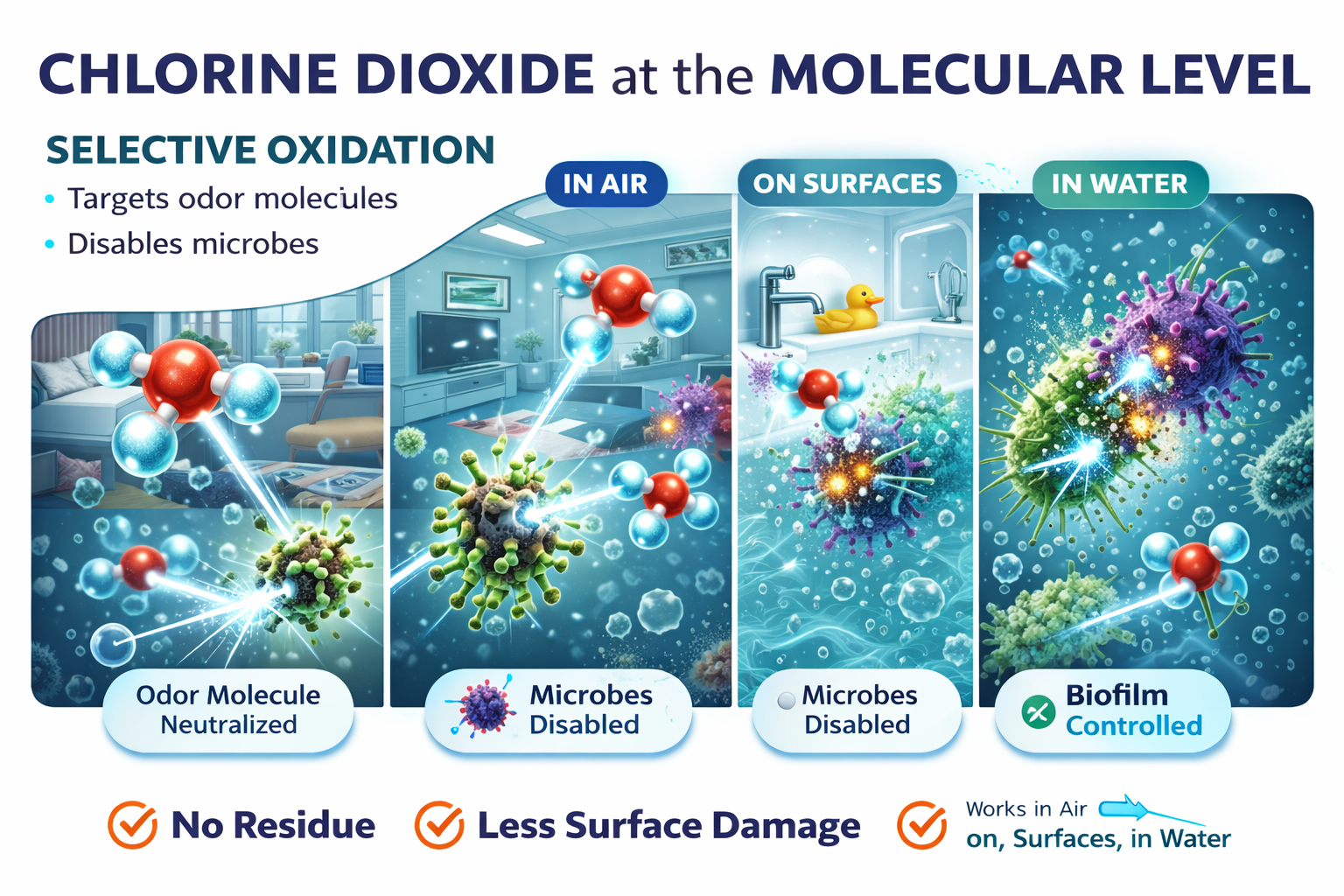
Chlorine dioxide (ClO₂) is unlike traditional disinfectants and deodorants. Rather than masking odors or relying on harsh surface chemistry, chlorine dioxide works at the molecular level, neutralizing odor-causing compounds and disabling microbes through a highly efficient process known as selective oxidation.
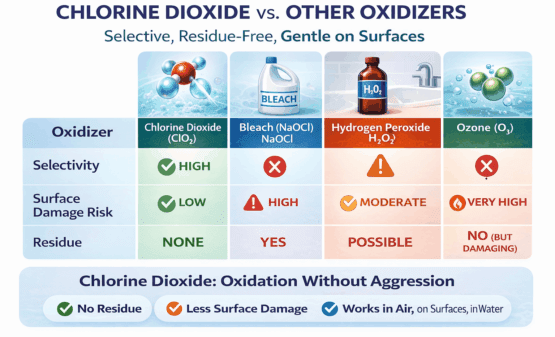
This unique mode of action allows chlorine dioxide to be effective in air, on surfaces, and in water—while leaving no harmful residue and causing significantly less damage to materials than other oxidizers such as bleach, hydrogen peroxide, or ozone.
This page explains why chlorine dioxide works the way it does, how it differs from traditional disinfectants, and what makes it uniquely suited for modern cleaning and odor control.
Most disinfectants work in one of two ways:
- Broad chemical aggression (bleach, strong acids, ozone)
- Surface disruption (quats, alcohols, soaps)
Chlorine dioxide works differently.
- Rather than indiscriminately reacting with everything it touches, chlorine dioxide targets specific molecular structures commonly found in microbes and odor compounds. This targeted behavior is why ClO₂ is described as a selective oxidizer.
A Gas with Precision, Not Force
Chlorine dioxide exists as a true dissolved gas, not an ionic solution like bleach. This allows it to:
- Penetrate biofilms and porous materials
- Move freely through air and water
- Reach hidden odor sources and microbial reservoirs
In short, ClO₂ goes where microbes and odors hide—instead of only treating exposed surfaces.
Understanding Oxidation as a Mode of Action
To understand why chlorine dioxide is so effective, it helps to understand oxidation.
What Is Oxidation?
Oxidation is a chemical process in which electrons are removed from a molecule. When this happens to:
- Odor molecules → their structure is altered so they can no longer produce smell
- Microbial cells → critical proteins and enzymes stop functioning
Unlike detergents (which lift and remove), oxidation chemically neutralizes the problem.
Selective Oxidation — The Key Advantage of Chlorine Dioxide
What Does “Selective Oxidation” Mean?
Selective oxidation means chlorine dioxide reacts only with specific electron-rich compounds, such as:
- Sulfur compounds (common in odors)
- Amines (urine, decay, smoke smells)
- Phenols and volatile organic compounds (VOCs)
- Critical amino acids in microbial proteins
It does not aggressively react with most plastics, metals, fabrics, sealants, or finished surfaces.
This is the fundamental reason chlorine dioxide can:
- Eliminate odors instead of masking them
- Kill microbes without damaging substrates
- Leave no residue or toxic byproducts
How Chlorine Dioxide Neutralizes Odor Molecules & Microbes
Odors are not “smells” — they are volatile molecules floating in air or embedded in materials.
Odor Destruction vs Odor Masking
Chlorine dioxide reacts directly with odor-causing molecules, breaking the bonds that allow them to be detected by the human nose. Once oxidized, the molecule no longer exists as an odor.
This is why ClO₂ is effective against:
- Smoke and fire odors
- Mold and mildew smells
- Urine and pet odors
- Food, grease, and organic decay odors
Microbial Inactivation at the Protein Level
Microbes rely on enzymes and proteins to survive. Chlorine dioxide selectively oxidizes:
- Sulfur-containing amino acids
- Tyrosine residues
- Critical metabolic enzymes
This causes rapid loss of cellular function, leading to microbial death or inactivation.
Importantly, chlorine dioxide does not need to rupture the cell wall, which makes it effective even against microbes that resist other disinfectants.
No Residue — Why Chlorine Dioxide Leaves Surfaces Clean
Unlike bleach or quaternary disinfectants, chlorine dioxide:
- Does not form sticky films
- Does not leave active chemical residues
- Breaks down into trace chloride and oxygen
Why Residue Matters
Residues can:
- Attract dirt
- Interfere with finishes
- Cause long-term material degradation
- Create re-odor problems
Because chlorine dioxide leaves nothing behind, surfaces remain neutral, clean, and safe for re-occupancy.
A Quick Summary: What's to Remember Here
- Chlorine dioxide works by selective oxidation
- It destroys odors and microbes at the molecular level
- It leaves no residue
- It causes less damage to surfaces than other oxidizers
- It works effectively in air, on surfaces, and in water
- It solves problems rather than covering them up
One Technology, Three Environments — Air, Surfaces & Water
As a gas, chlorine dioxide:
- Moves throughout enclosed spaces
- Reaches behind walls, fabrics, and HVAC pathways
- Neutralizes airborne microbes and odors at the molecular level
This makes it uniquely effective for whole-room deodorization.
When dissolved in water:
- ClO₂ remains active as a gas in solution
- Does not bind to surfaces or leave films
- Continues working until it reacts with a target molecule
Ideal for:
- Touchpoint disinfection
- Porous and non-porous surfaces
- Restoration and odor remediation
In water systems, chlorine dioxide:
- Controls biofilms
- Disinfects without forming chlorinated byproducts
- Remains effective across a wide pH range
This is why ClO₂ is widely used in municipal water treatment and industrial sanitation.

P.O. Box 34 Winterport, Maine 04496
If you need more information, or are a commercial buyer, please visit this parent site.

